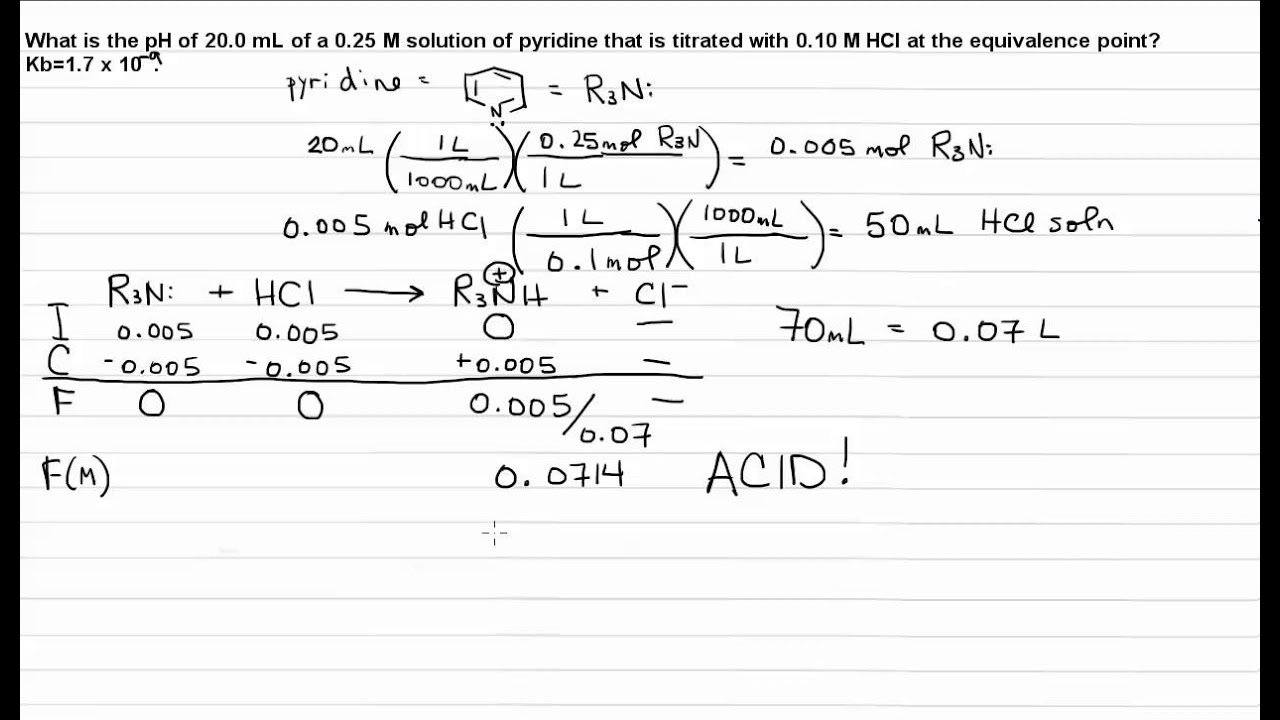
Calculate the Ph at the Equivalence Point
As the equivalence point is approached the pH drops rapidly before leveling off at a value of about 070 the pH of 020 M HCl. Calculate the pH at the equivalence point for the following titration.
Titration Weak Base Strong Acid Equivalence Point Youtube
The pH at the equivalence point for the titration will be 065.
. The pH is then calculated using the expression. Calculate the pH at the equivalence point for the titration of 0120 M0120 M methylamine CH3NH2CH3NH2 with 0120 M HCl0120 M HCl. In the case of titration of weak acid with strong base pH at the equivalence point is determined by the weak acid salt hydrolysis.
CH₃NH₂ HCl CH₃NH₃ Cl. Calculate the pH at the equivalence point in the titration of 500 mL of 0120 M methylamine K5 44 x 10- with 0335 M HCI. Calculate the pH at the equivalence point in titrating 0110 M solutions of each of the following acids with a solution 0090 M in NaOH.
Then apply the ICE approach. The K X b 44 10 4. A sodium hydroxide NaOH b hydroxylamineNH2OH c aniline C6H5NH2.
Using the pH of the solution a student determined that their hydroxide concentration was 01972 M. Since methyl-amine is a weak basecx so. Let the conjugate acid undergo hydrolysis.
Calculate the pH at the equivalence point of a titration between 500 mL unknown HNO2 concentration A. Solving for x we get. Therefore we can use the formula of weak base to calculate the OH-concentration at this equivalence point.
Turns out we require 62 mL or the CH3NH2 and 31 mL of the HCl for a total volume of 93 mL. Enter your answer in the provided box. D Calculate the pH at the equivalence point.
We can now substitute these back into the weak. Calculate the pH at the equivalence point for titrating0200 M solutions of each of the following bases with 0200M HBr. PH 700 Previous Answers Correct The molecular equation for the titration is as follows.
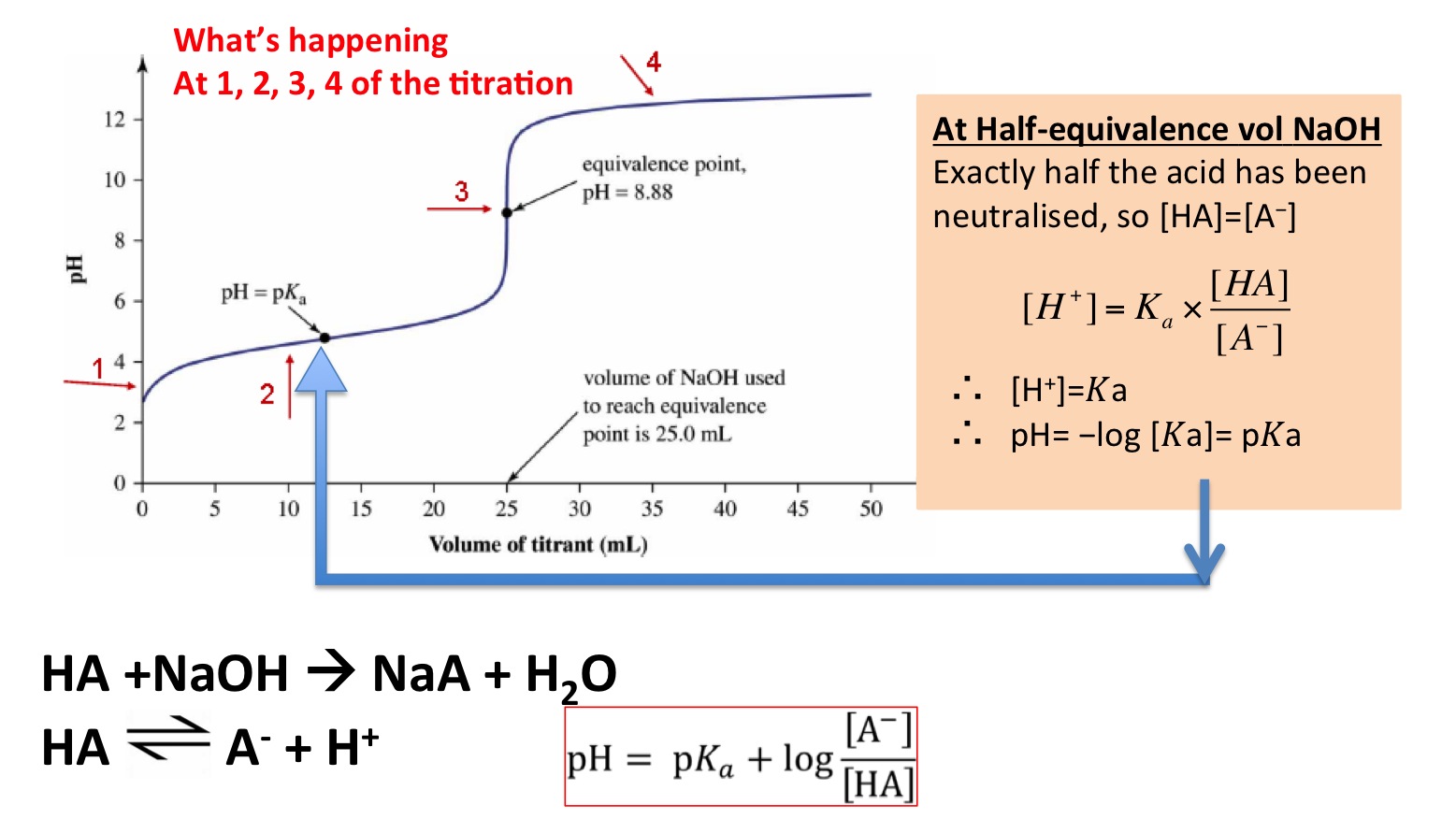
The titration of either a strong acid with a strong base or a strong base with a strong acid produces an S-shaped curve. See pH of weak acids and bases lecture and pH cheat sheet for details of calculation. Calculate the PH at equivalence point when a solution of 0.
To reach equivalence point HNO 3 KOH H 2 Ol KNO 3 aq I 0100 moles each sampletitrant C End After equivalence point any excess strong base KOH determines the pH. The pH of 01 M sodium acetate is calculated as follows. POH -logOH- excess pH 14 pOH Fig.
A weak base with pKb 608 with 3644 M HCl. Enter your answer in the provided box. CH₃NH₃ H₂O H₃O CH₃NH₂.
This video screencast was created with Doceri on an iPad. Since methyl-amine is a weak basecx so. X201 x 01 K_b12 746x10-6 OH- pOH -log746x10-6 513 pH 14 - pOH 887.
Item 4 A Review Constants Periodic Table Part A hydrobromnic acid HB Express your answer to two decimal places. At the equivalence point the moles of CH3NH2 equals the moles of HCl. Initial concentration of c 0230 M at eqm c-x x x.
CH3NH2 HCl 0210 M. Calculate the pH at the equivalence point for the titration of 020 M NH3 with 020 M HCl. I would assume you could use the henderson equation but I only get 498 which is wrong.
1 M K O H. That means we have to find pK b of conjugated base and calculate concentration of OH-starting from there then use pH14-pOH formula. Kb of ammonia is 18e-5 This is just a old test I am going over I managed to get a pH of 498.
1 M C H 3 C O O H is titrated with a solution of 0. Ka H₃O CH₃NH₂ CH₃NH₃ Since the given information is Kb lets find Ka in terms of Kb. Let the concentration of be x.
I 011 0 0. Solving for x we get. How do you calculate the pH at the equivalence point in a titration.
Solution for Calculate the pH at the equivalence point for the titration of 3644 M B. Therefore volumes will be equal which means at the equivalence point the concn of the salt will be 12 of 0210 or 0105 M. Initial concentration of c 0230 M at eqm c-x x x.
This is simple solution stoichiometry. C -x x x. E 011 - x x x.
010 M HCOOH and 010 M NaOH. CH3NH2 HCl CH3NH3Cl CH3NH3 HOH CH3NH2 H3O. In the case of titration of weak base with strong acid situation is very similar - pH at the equivalence point is determined by the weak base salt.
K b is not given but we can calculate it easily from ionic product of water K w and K a via the formula K w K aK b. K a of C H 3 C O O H 2 1 0 5 Medium. PH -logH At equivalence point moles of acid is equal to moles of base.
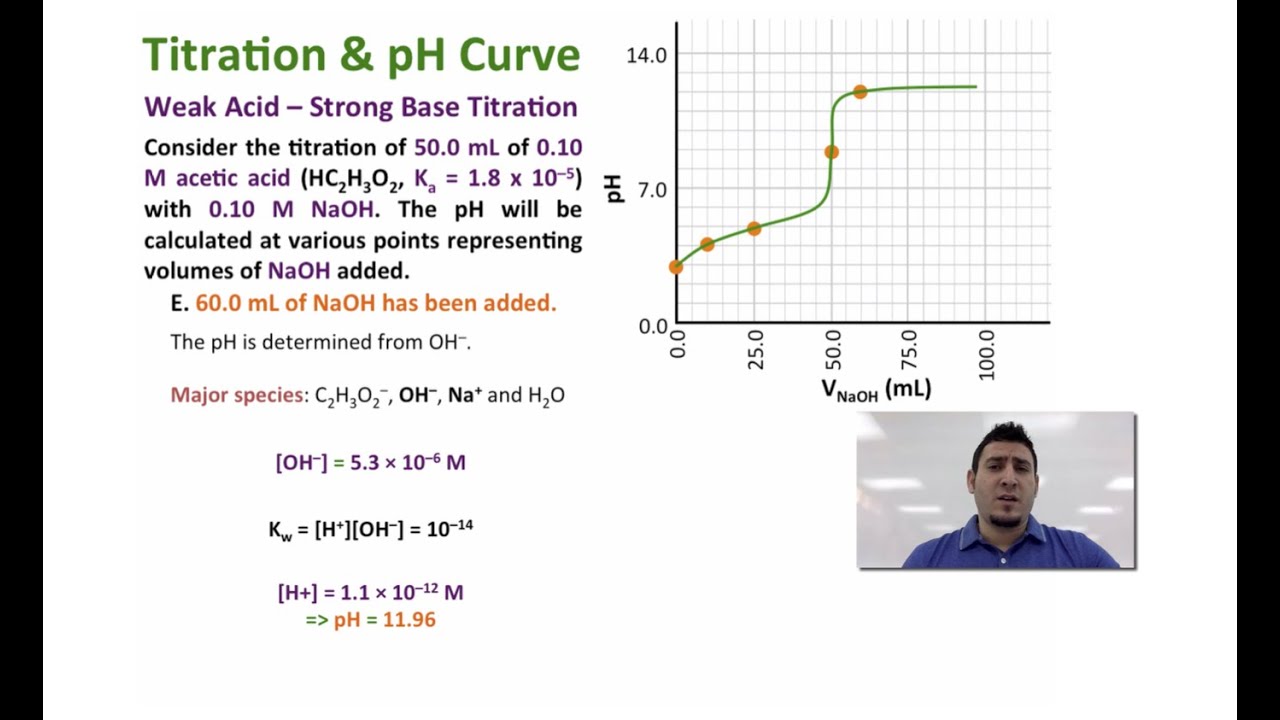
PH log H3O. HC 3H 5O 2 0275 mol L 0030 L 000825 mol NaOH 0200 mol L 004125 L 000825 mol I 00825 00825 0 - E 0 0 000825 mol - C 3H 5O 2 000825 mol 007125 L 0116 M H 2O C 3H 5O 2 ä OH HC 3 H 5 O 2 initial 0116 0 0 change x x x x C 3H 5O 2R. Chemistry questions and answers.
Let the concentration of be x. Concentration of CH 3 COO-can also be easily calculated keeping in mind the total volume is 50cm 3. The pH at the equivalence point for the titration will be 065.
K_b 556x10-10 OH-HAA- x201-x. Calculate the pH at the equivalence point of a titration of 62 mL of 01 M C H X 3 N H X 2 with 020 M HCl. If total KOH added was 0150 moles then excess OH- 0050 moles.
Doceri is free in the iTunes app store. But that is not right the right answer is 512 and I cant figure out why.

Ib Chemistry Higher Level Notes Acid Base Calculations

Weak Acid Strong Base Titration Ph After Equivalence Point Youtube
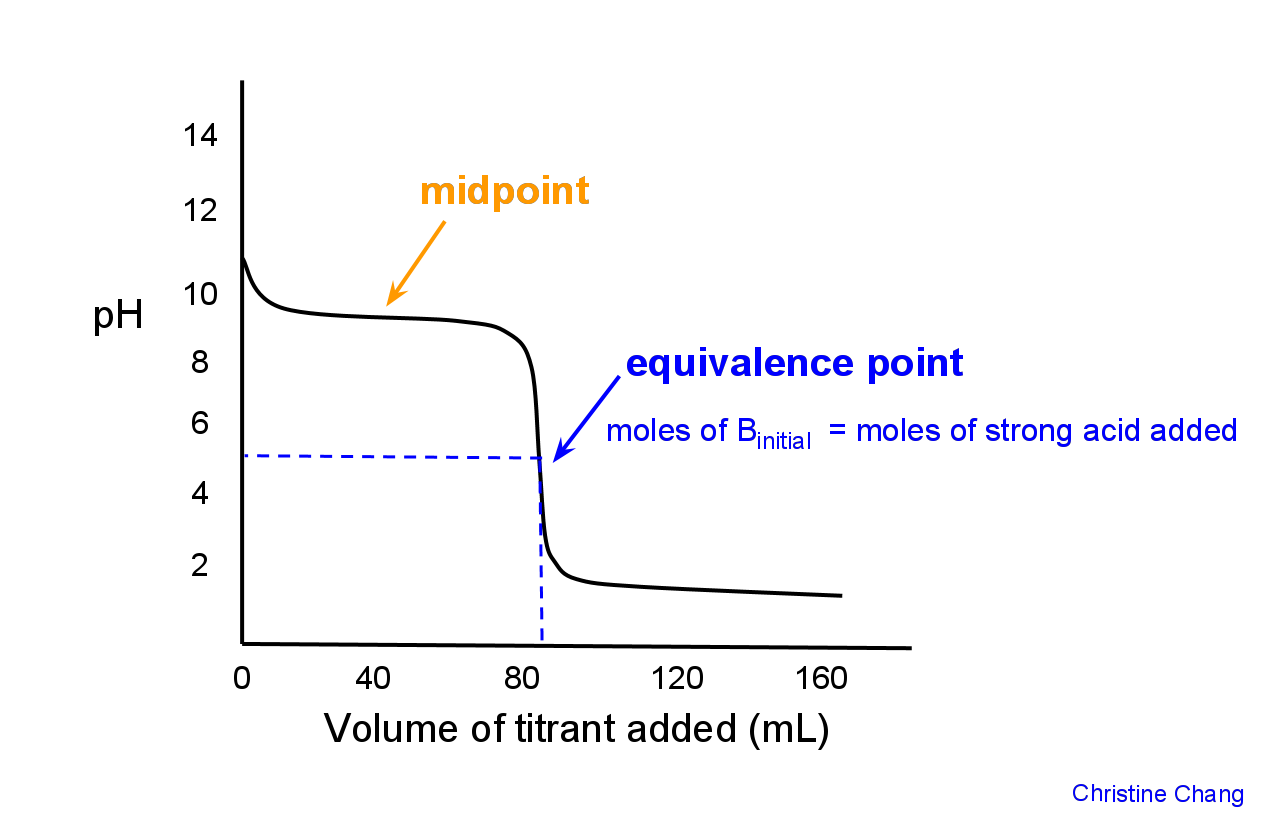
Titration Of A Weak Base With A Strong Acid Chemistry Libretexts

Strong Acid Strong Base Titration Ph Before Equivalence Point Youtube

The Ph At One Half The Equivalence Point In An Acid Base Titration Was Found To Be 5 67 What Is The Value Of K A For This Unknown Acid Socratic
How Do You Calculate The Ph At The Equivalence Point For The Titration Of 190m Methylamine With 190m Hcl The Kb Of Methylamine Is 5 0x10 4 Socratic
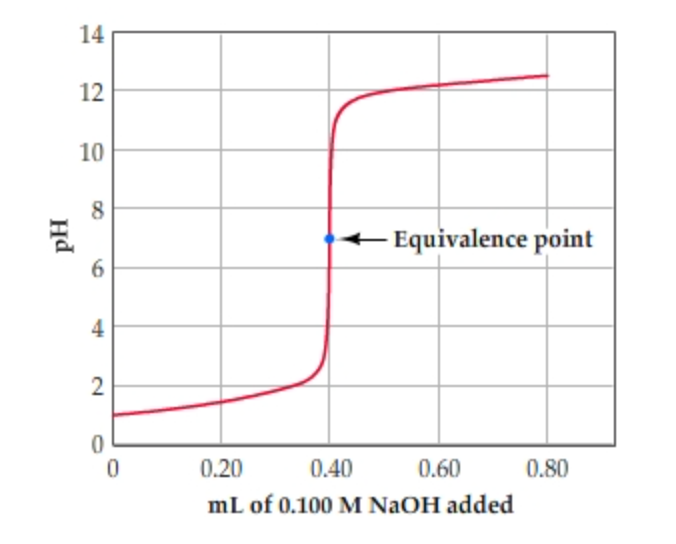
Solved Learning Goal To Calculate The Ph At The Equivalence Chegg Com

Calculate The Ph At One Half The Equivalence Point Youtube
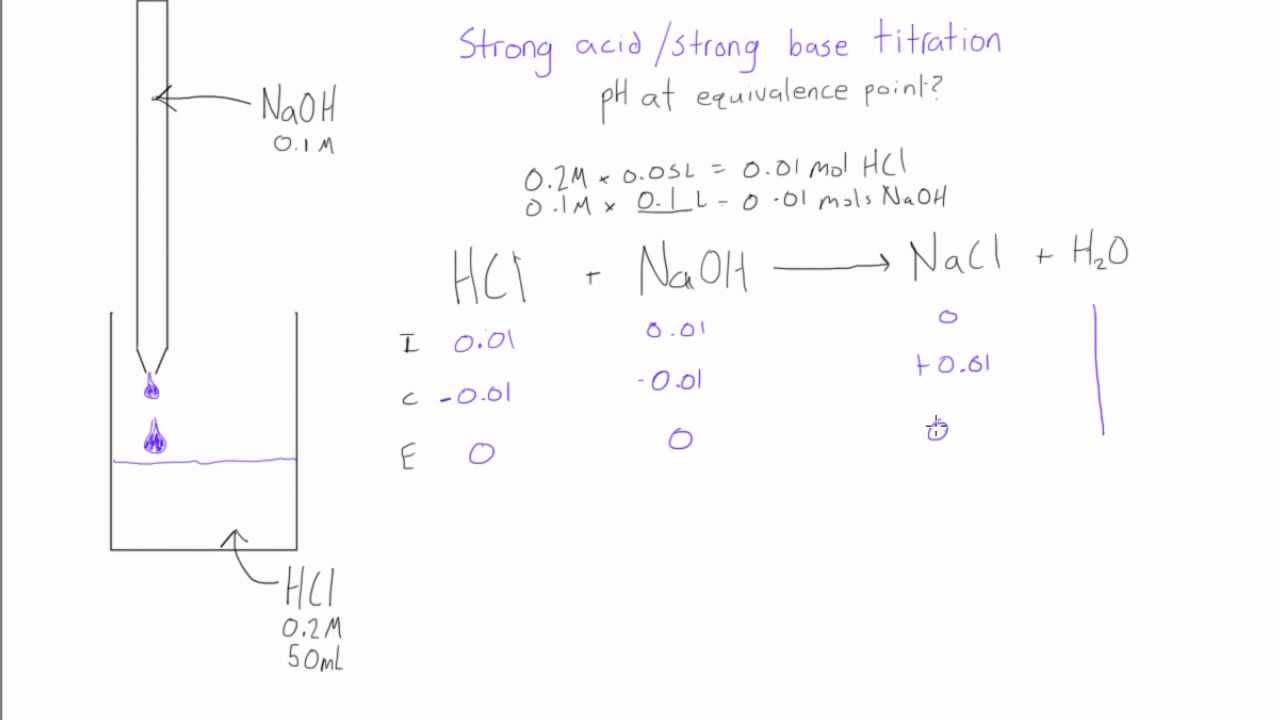
Strong Acid Strong Base Titration Ph At Equivalence Point Youtube
Comments
Post a Comment